Extracellular membrane vesicles as spherical particles secreted from cells of both prokaryotic and eukaryotic origin. In bacteria, these membrane vesicles (MVs) are 50 to 250 nm in size and they are encapsulated by either a double or single cell membrane. Their cargo contain a variety of bacterial components including proteins and nucleic acids thereby representing the mother cell but in a non-replicative form. Their production in bacteria are known to be associated with a range of phenotypes including biofilm formation, horizontal gene-transfer, toxin delivery, virulence and cell-cell communication. We are interested in studying the impact MVs have in host-pathogen interactions and investigating their potential use as a vaccine in fish species important for aquaculture and production animals.
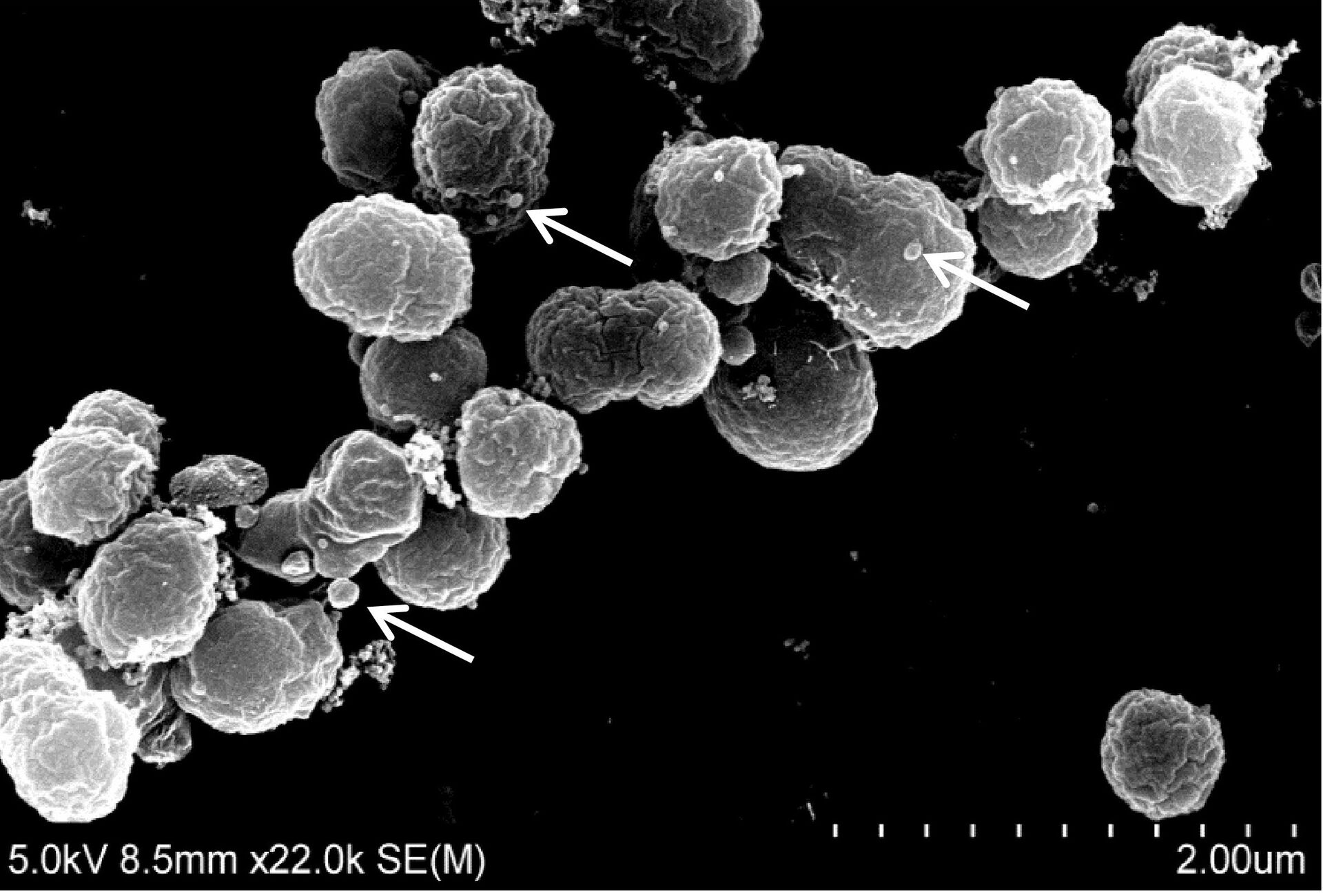
Photo: Julia I Tandberg and Eva Berger.
Subprojects
Novel vesicle-based vaccine platform for animal health

Treatment of bacterial infections may lead to an expanded use of antibiotics, with increased risk of antimicrobial resistance development. Vaccination is one way of reducing the use of antimicrobial therapy also within animal health. Bacteria that reside inside cells are particularly challenging to vaccinate against as stimulation of both humoral and cellular immune responses is needed. Many disease-causing bacteria secrete vesicles that are considered promising vaccine candidates against various bacterial diseases including intracellular pathogens. A disadvantage of vesicles vaccines is that it suffers from low production yields. In this project we try to develop an Escherichia coli based vesicle vaccine platform that will enable high yields and low-cost vaccine manufacturing against diseases that plague the livestock and aquaculture sector.
Collaborators: Aina Rengmark (INVEN2).
Funding: SPARK

Membrane vesicles as vaccines in aquaculture
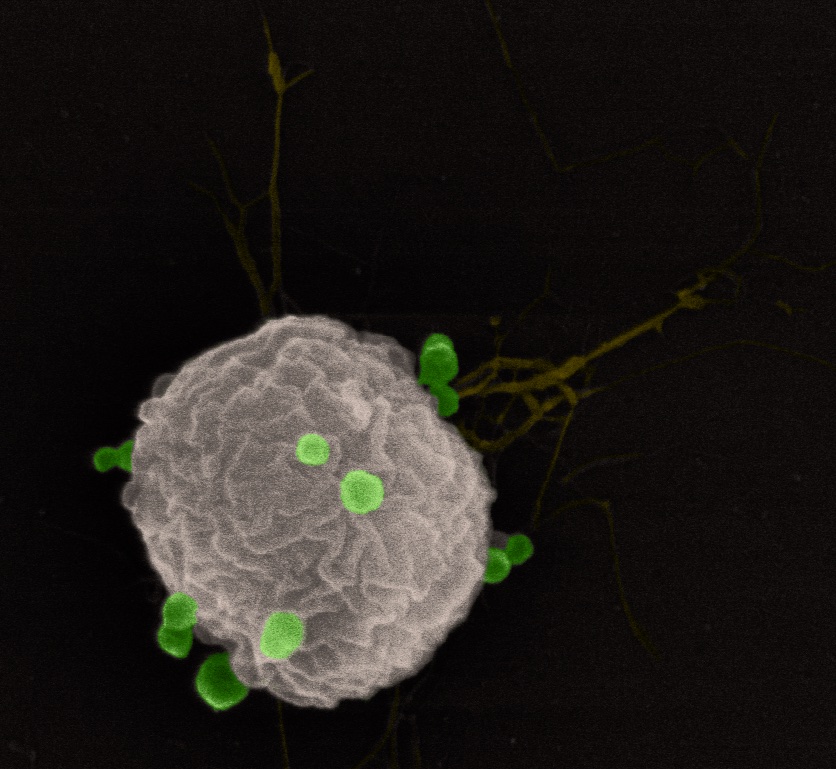
We are interested in the MVs use as a vaccine against infections caused by intracellular pathogens in aquaculture. Such disease are, because of the intracellular nature difficult to vaccinate against as both a humoral and cellular immune response are generally needed to elicit for protection. MVs has been shown to elicit protection against several infectious disease in a range of animals and has successfully been used as a vaccines against meningitides in humans. In our group we are investigated the vaccine potential of MVs against infections caused by the bacterial pathogens Francisella noatunensis subsp noatunensis, Francisella orientalis, Piscirickettsia salmonis and Yersinia ruckeri. For this we use both fish cell lines as primary investigations for cell immune responses, as well as a zebrafish vaccine and infection model. With positive results in these models we test our vaccine candidates in the natural hosts the Atlantic salmon, Atlantic cod, rainbow trout and tilapia.
Collaborations: Prof. Dirk Linke (Department of Biosciences, UiO) Prof. Henning Sørum (Norwegian University of Life Sciences (NMBU)), Dr. Duncan Colquhoun (Norwegian Veterinary Institute), Dr. Håvard Sletta (SINTEF Biotechnology) and Aina Rengmark (Inven2: The UiO Technology Transfer Office). The project has been performed in communication with the industrial partners MOWI, one of Norway largest fish farming companies, and Vaxxinova, a vaccine company within animal health.
Funding: The projects has been funded by The Research Council of Norway through the Biotek2020 program, FORNY program, Havbruk program and Milepæl program, as well as the UiO.
![]()
![]()
Bacterial membrane vesicle biology in host-pathogen interactions
Membrane vesicles secretion are associated with several important phenotypes for host-pathogen interaction including antimicrobial resistance, cell-cell communication and delivery of cargo into host cells. To get a better understanding of these processes we are exploring the impact of MV cargo in bacterial functions. We have been using a more holistic approach where both the protein and genetic cargo (both DNA and RNA) of the MVs has been compared to that of the mother cell. We have mainly used the bacterium Vibrio cholerae as models, and we are using cell lines in order to study what impact the MV cargo may have in host cellular responses. Some of our major findings so far are that DNA with certain TA-rich regions were more prone to packing into the MV together with shorter rather than longer RNA sequences, and that post-secretion translation is limited in the MVs.
Collaboration: Dr. Anders Krabberød, Department of Biosciences, UiO.
Funding: This work is supported mainly by UiO through CiME, Center of Integrative Microbial Evolution, but also the RCN FORNY program.
Membrane vesicle nano-particle vaccine formulations
Isolated from Gram-negative bacteria membrane vesicles based formulation naturally contain substantial amount of the lipopolysaccharide (LPS) endotoxin that could potentially be harmful to the host upon exposure. Ways to solve this problem is i.e chemically remove the LPS, or to create mutant strains in the LPS biogenesis pathways. As the bacterial membrane vesicles are only in nano-meter size ratio, we have formulated the MVs into nano-capsules and are testing their vaccine potential in our zebrafish vaccine and infection model. This MV nano-particle vaccine formulation seem to elicit little toxicity and protection in our model.
Collaborations: We are in this project collaborating with Prof. Marianne Hiorth, expert researchers in pharmaceutical sciences at Department of Pharmacy, UiO.
Funding: UiO and RCN FORNY program.