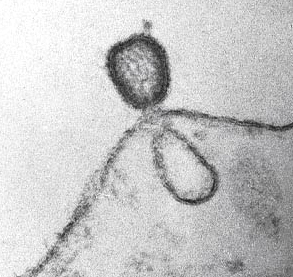
Viruses are mobile genetic elements with both intracellular and extracellular forms, totally dependent on a host cell for their replication. Many viral infections are chronic and can be regarded as a part of the host metagenome where viral and host genes balance each other. In contrast, during acute infections there is an ongoing battle between host immunity and viral replication resulting in viral clearance or death of the host. Both prokaryotic and eukaryotic cells have developed molecular machinery for viral detection and clearance (Goldstein and Scull, 2022; Kawai and Akira, 2008; Novoa et al., 2010; Rathinam and Fitzgerald, 2010; Yoneyama and Fujita, 2007) .
Today, fish and shellfish aquaculture produce about 50% of total seafood for human consumption. Bacterial and viral infections constitute a constant stress to animal welfare and economics of the industry. Several bacterial diseases have been reduced to a minimum due to efficient vaccination programs, but many viral diseases still pose a great problem. To develop better profylaxis (vaccines) for these diseases we need a better understanding of the pathogen – host interactions for marine viruses like ISAV, IPNV, SAV and others (Evensen et al., 2005).
In our lab we investigate the transcriptional and biochemical changes occurring in cells and tissues from Atlantic salmon infected with viruses . Using RNAseq, QPCR and other techniques we try to understand how cells contain viral replication (innate immunity) and the mechanisms used by the viruses to circumvent this cellular defense apparatus. The immune system of teleost fish consist of both an innate and adaptive response like in mammals, but the specificity and affinity of antibodies produced after infection or immunization are lower. Efficient cytotoxic T-cell responses (CTL) have also been hard to demonstrate. To combat viral infections, fish therefore seems to rely more on the germline-encoded pattern recognition receptors (PPRs) like TLR, RIG-I and Mda5 to detect virus and induce antiviral responses (Workenhe et al., 2010). These responses include secretion of alarm molecules like interferons and interleukins (Robertsen, 2006, 2008)(putting neighbouring cells in an antiviral state), and synthesis of effector molecules like Mx, ISG15 and PKR to restrict the replication of the intracellular viral pathogen (Haller et al., 2007). We are also interested in the effects of dietary omega n-3 fatty acids and how adipose tissue biochemical and cellular composition influence these processes (Andresen et al., 2019; Arnemo et al., 2017a; Arnemo et al., 2017b).
Using tools of functional genomics we have identified many transcripts induced during in vitro infection with infectious salmon anemia virus (Schiøtz et al., 2008) (Andresen et al., 2020). We have used these data to study the transcriptional changes in greater detail during the infectious process in vitro (Jorgensen et al., 2007), whereas others have followed up these studies in vivo (Jørgensen et al., 2007). When animals are infected with virus, one mechanism of containing the infection is by induction of controlled cell death (apoptosis) in the infected cells. This “sacrifice” is can be seen a protective measure to control infection and many viruses have developed anti-apoptotic proteins to counter this prosess (Koyama et al., 1998). When salmon cells are infected with ISAV, cell- and strain-dependent apoptotic changes take place and we have investigated the role of this process in immune responses to ISAV (Schiotz et al., 2009). ISAV also induce autophagy in salmon cells but it is presently unclear if this is a part of the protective response, or used by the virus to increase replication (Schiotz et al., 2010).
Andresen, A.M.S., Boudinot, P., Gjoen, T., 2020. Kinetics of transcriptional response against poly (I:C) and infectious salmon anemia virus (ISAV) in Atlantic salmon kidney (ASK) cell line. Dev Comp Immunol 110, 103716.
Andresen, A.M.S., Lutfi, E., Ruyter, B., Berge, G., Gjøen, T., 2019. Interaction between dietary fatty acids and genotype on immune response in Atlantic salmon (Salmo salar) after vaccination: A transcriptome study. PloS one 14, e0219625-e0219625.
Arnemo, M., Kavaliauskis, A., Andresen, A.M.S., Bou, M., Berge, G.M., Ruyter, B., Gjoen, T., 2017a. Effects of dietary n-3 fatty acids on Toll-like receptor activation in primary leucocytes from Atlantic salmon (Salmo salar). Fish Physiol Biochem 43, 1065-1080.
Arnemo, M., Kavaliauskis, A., Andresen, A.M.S., Bou, M., Berge, G.M., Ruyter, B., Gjøen, T., 2017b. Effects of dietary n-3 fatty acids on Toll-like receptor activation in primary leucocytes from Atlantic salmon (Salmo salar). Fish Physiology and Biochemistry 43, 1065-1080.
Evensen, O., Brudeseth, B., Mutoloki, S., 2005. The vaccine formulation and its role in inflammatory processes in fish--effects and adverse effects. Dev Biol (Basel) 121, 117-125.
Goldstein, M.E., Scull, M.A., 2022. Modeling Innate Antiviral Immunity in Physiological Context. J Mol Biol 434, 167374.
Haller, O., Staeheli, P., Kochs, G., 2007. Interferon-induced Mx proteins in antiviral host defense. Biochimie 89, 812-818.
Jørgensen, S.M., Hetland, D.L., Press, C.M., Grimholt, U., Gjøen, T., 2007. Effect of early infectious salmon anaemia virus (ISAV) infection on expression of MHC pathway genes and type I and II interferon in Atlantic salmon (Salmo salar L.) tissues. Fish & shellfish immunology 23, 576-588.
Jorgensen, S.M., Syvertsen, B.L., Lukacs, M., Grimholt, U., Gjoen, T., 2007. "Expression of MHC class I pathway genes in response to infectious salmon anaernia virus in Atlantic salmon (Salmo salar L.) cells" (vol 21, pg 548, 2006). Fish & shellfish immunology 22, 734-734.
Kawai, T., Akira, S., 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci 1143, 1-20.
Novoa, B., Mackenzie, S., Figueras, A., 2010. Inflammation and Innate Immune Response Against Viral Infections in Marine Fish. Curr Pharm Des.
Rathinam, V.A., Fitzgerald, K.A., 2010. Inflammasomes and anti-viral immunity. J Clin Immunol 30, 632-637.
Robertsen, B., 2006. The interferon system of teleost fish. Fish & shellfish immunology 20, 172-191.
Robertsen, B., 2008. Expression of interferon and interferon-induced genes in salmonids in response to virus infection, interferon-inducing compounds and vaccination. Fish & shellfish immunology 25, 351-357.
Schiotz, B.L., Baekkevold, E.S., Poulsen, L.C., Mjaaland, S., Gjoen, T., 2009. Analysis of host- and strain-dependent cell death responses during infectious salmon anemia virus infection in vitro. Virol J 6, 91.
Schiøtz, B.L., Jørgensen, S.M., Rexroad, C., Gjøen, T., Krasnov, A., 2008. Transcriptomic analysis of responses to infectious salmon anemia virus infection in macrophage-like cells. Virus Research 136, 65-74.
Schiotz, B.L., Roos, N., Rishovd, A.-L., Gjoen, T., 2010. Formation of autophagosomes and redistribution of LC3 upon in vitro infection with infectious salmon anemia virus. Virus Research 151, 104-107.
Workenhe, S.T., Rise, M.L., Kibenge, M.J.T., Kibenge, F.S.B., 2010. The fight between the teleost fish immune response and aquatic viruses. Molecular Immunology 47, 2525-2536.
Yoneyama, M., Fujita, T., 2007. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem 282, 15315-15318.