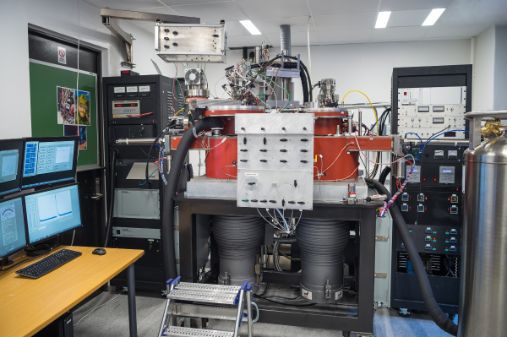
TAP-3E instrument at the Department of Chemistry, University of Oslo is a part of the Norwegian National Surface and Interface Characterisation Laboratory (NICE).
Principles of operation
TAP is a time-resolved technique for precise kinetic characterization of gas-solid reactions in catalysis, adsorption, and other applications. Unlike in conventional kinetic devices (e.g. well-mixed or tubular flow reactors), gas transport in the TAP reactor occurs via Knudsen diffusion – a special transport regime whereby molecules collide with the reactor walls and catalytic surfaces, but not with each other. Knudsen diffusion is advantageous as a transport regime for kinetic characterization because it is very well-defined, i.e. the transport rate of each gas is uniquely defined by its molecular mass, the temperature, and the geometry of the reactor packing. Gas-phase reactions which may interfere with the gas-solid interactions of interest are completely eliminated under TAP conditions. Importantly, both model and realistic high-surface area materials can be studied using TAP.
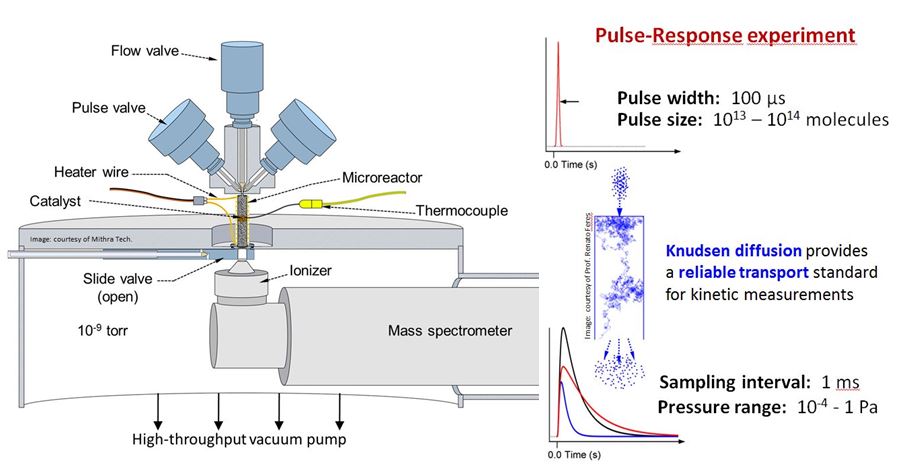
In the beginning of a typical TAP pulse-response experiment, a small amount (10-9 mol/pulse) of gas mixture with known composition is rapidly injected into an evacuated (10-7 torr background) microreactor with a catalytic sample. After traversing the reactor space by Knudsen diffusion, the unreacted and newly formed molecules escape into the adjacent vacuum chamber where they are quantitatively detected by a calibrated mass spectrometer. The millisecond time resolution of these kinetic data provides unique insight into the inner workings of gas-solid reactions. Our TAP instrument is equipped with both Quadrupole (QMS) and Time-of-Flight (ToF) detectors for enhanced mass-and time-resolution. Pretreatments and kinetic measurements under atmospheric pressure flow conditions are also possible within the same instrument, and rapid switching between flow and pulsing modes enables complex experimental design.
Current projects
- Diffusion characterization in microporous and hierarchically porous materials (zeolites, zeotypes, MOFs);
- Intrinsic kinetics of olefin methylation and other steps of the Methanol-To-Hydrocarbons (MTH) chemistry in zeolites;
- CO2 reduction by non-stoichiometric ceria and other mixed oxides, generation and stability of oxygen vacancies in metal oxides
- Tandem spectro-kinetic (TAP-XPS) investigations of reaction mechanisms on complex metal oxides
Recent publications
Vladyslav Shostak, Evgeniy Redekop, and Unni Olsbye, Parametric sensitivity analysis of the transient adsorption-diffusion models for hydrocarbon transport in microporous materials, Catalysis Today, 2023, 417, 113785 DOI: 10.1016/j.cattod.2022.05.050
Evgeniy A. Redekop, Gregory S. Yablonsky, and John T. Gleaves, Truth is, we all are transients: A perspective on the time-dependent nature of reactions and those who study them, Catalysis Today, 2023, 417, 113761 DOI: 10.1016/j.cattod.2022.05.026
Javier Narciso, Enrique V. Ramos-Fernandez, José J. Delgado-Marín, Christopher W. Affolter, Unni Olsbye, Evgeniy A. Redekop, New route for the synthesis of Co-MOF from metal substrates, Micropor. Mesopor. Mat., 2021, 324, 111310, DOI: 10.1016/j.micromeso.2021.111310
Evgeniy A. Redekop, Niclas Johansson, Esko Kokkonen, Samuli Urpelainen, Felipe Lopes da Silva, Mikko Kaipio, Heta-Elisa Nieminen, Foqia Rehman, Ville Miikkulainen, Mikko Ritala, and Unni Olsbye, Synchronizing gas injections and time-resolved data acquisition for perturbation-enhanced APXPS experiments, Review of Scientific Instruments, 2021, 92, 044101, DOI: 10.1063/5.0039957
Pieter Cnudde, Evgeniy A Redekop, Weili Dai, Natale G. Porcaro, Michel Waroquier, Silvia Bordiga, Michael Hunger, Landong Li, Unni Olsbye, Veronique Van Speybroeck, Experimental and theoretical evidence for promotional effect of acid sites on the diffusion of alkenes through small‐pore zeolites, Angew Chem Int Ed., 2021, DOI: 10.1002/anie.202017025
Evgeniy A. Redekop, Andrea Lazzarini, Silvia Bordiga, Unni Olsbye, A temporal analysis of products (TAP) study of C2-C4 alkene reactions with a well-defined pool of methylating species on ZSM-22 zeolite, J. Catal., 2020, 385, 300-312, DOI: 10.1016/j.jcat.2020.03.020
Ping Liu, Evgeniy A. Redekop, Xiang Gao, Wen-Chi Liu, Unni Olsbye, Gabor A. Somorjai, Oligomerization of Light Olefins Catalyzed by Brnsted-Acidic Metal Organic Framework-808, J. Am. Chem. Soc., 2019, 141, 29, 11557-11564, DOI: 10.1021/jacs.9b03867
People
PIs: Unni Olsbye, Evgeniy Redekop, Postdoctoral fellow: Juan Ignacio Mirena, PhD candidate: Vladyslav Shostak
Technical specialists: Terje Grønås, Behzad Foroughinejad, Tomas Mikoviny, Simen Kristiansen, Kjell Rune Lind, Jonas Ringnes, Maren Charlotte Lithun, Anthony D. L. Hansen, Hans Borg, Arvid Andreassen, Elijah Jeremiah Aller
Collaborations
Veronique Van Speybroeck (CMM, University of Gent, Belgium), Samuli Urpelainen (University of Oulu, Finland), Esko Kokkonen (MAX-IV, Lund University, Sweden), Anibal Boscoboinik (Brookhaven National Laboratory, USA), John Gleaves (WUSTL, Mithra Tech., USA), John Gleaves Jr. (Mithra Tech., USA), Gregory Yablonsky (Saint Louis University, USA), Rebecca Fushimi (INL, USA), Yves Schuurman (CNRS Lyon, France), Guy B. Marin (LCT, University of Gent, Belgium), Vladimir Galvita (LCT, UGENT, Belgium), Denis Constales (UGENT, Belgium), Anatoly Frenkel (Stony Brook University, USA), Renato Feres (WUSTL, USA), Alexandru Botan (DTU Energy, Denmark), Benoit Cordonnier (UiO, Geosciences, Norway), Bob Madix (Harvard University, USA), Cynthia Friend (Harvard University, USA), Christian Reece (Rowland Institute at Harvard, USA), Gabor Somorjai (UC Berkeley, LBNL, USA), Francisco Javier Narciso Romero (Universidad de Alicante, Spain)
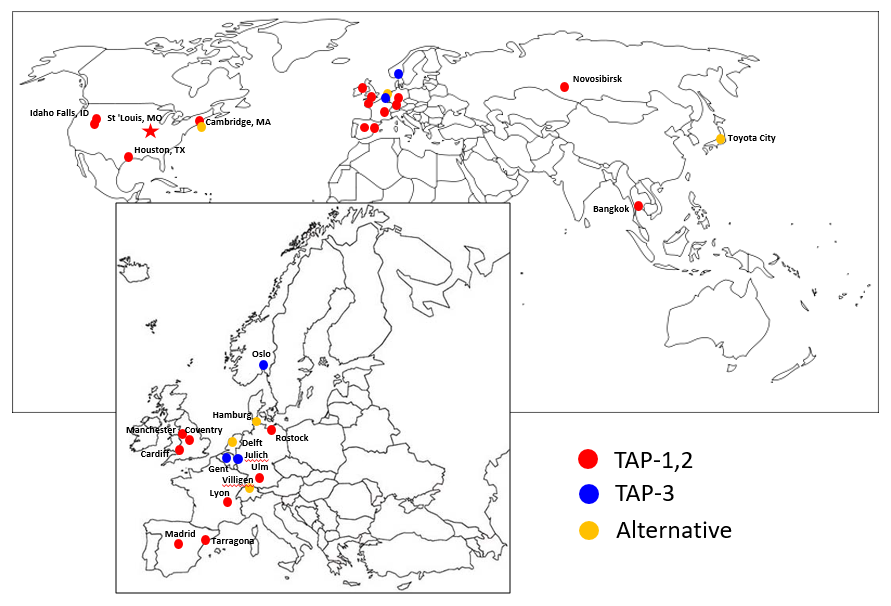
Global TAP community
TAP-team at UiO is proud to be part of a larger international TAP community.
References and links
National Surface and Interface Characterisation Laboratory (NICE)