Bacterial infections have historically killed more people than any other disease, and diarrheal disease is the second leading cause of death in small children. Cholera, which is caused by the human pathogen Vibrio cholerae, gives rise to the most severe form of secretory diarrhea. A similar, but milder disease is traveler's diarrhea, caused by enterotoxigenic Escherichia coli (ETEC).
About the project
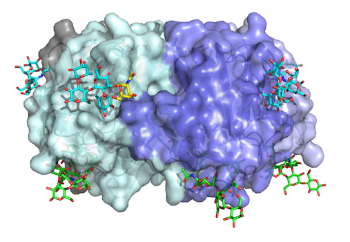
The major virulence factors of V. cholerae and ETEC are the cholera toxin (CT) and heat-labile enterotoxin (LT), respectively. These two toxins are structurally as well as functionally related and consist of one enzymatically active A-subunit, non-covalently anchored in the center of a receptor-binding homo B-pentamer (shown in the Figure above). Our main interest relates to the receptor specificity of these toxins, which may explain the blood group association of disease. We are also curious why ETEC is a much milder disease than cholera? Finally, we want to understand the activation mechanism of the toxins by host chaperones.
The first step of infection is mediated by bacterial colonization factors. Here, our focus is on the adhesin GbpA and related colonization factors from other bacteria, which all share a catalytic domain with lytic polysaccharide monooxygenase (LMPO) activity. This enzymatic activity makes the proteins highly relevant as catalysts for the generation of sustainable fuels from industry waste products like cellulose and chitin (Costa et al., 2019).
Research Highlights
Cholera toxin and blood-group dependence
In collaboration with the groups of Susann Teneberg and Jonas Ångström in Gothenburg, Sweden, we solved the crystal structure of a toxin hybrid with increased binding affinity to blood group antigens, revealing the presence of a second binding site on the toxin (Holmner et al., 2004). Subsequently, we could show that also the parent toxins bind to blood group antigens (Holmner et al., 2007 and Heggelund et al., 2016), which led to a breakthrough in our understanding of cholera blood group dependence (Holmner et al., 2010; Heggelund et al., 2016; Heim et al. 2019). The main point is that blood group 0 antigens bind more strongly to the toxins than determinants of blood groups A and B, involving two alternative orientations. These results may be used to tailor treatments based on a patient's blood group, or for making a new drug that for example blocks the toxin binding to the intestinal cells. In fact, we started to work on such cholera prophylactics in collaboration with the group of Anna Bernardi (Heggelund et al., 2017a). – Those interested in knowing more about the role of histo-blood group antigens as mediators of infections, are referred to a review we wrote together with Annabelle Varrot and Anne Imberty (Heggelund et al., 2017b).
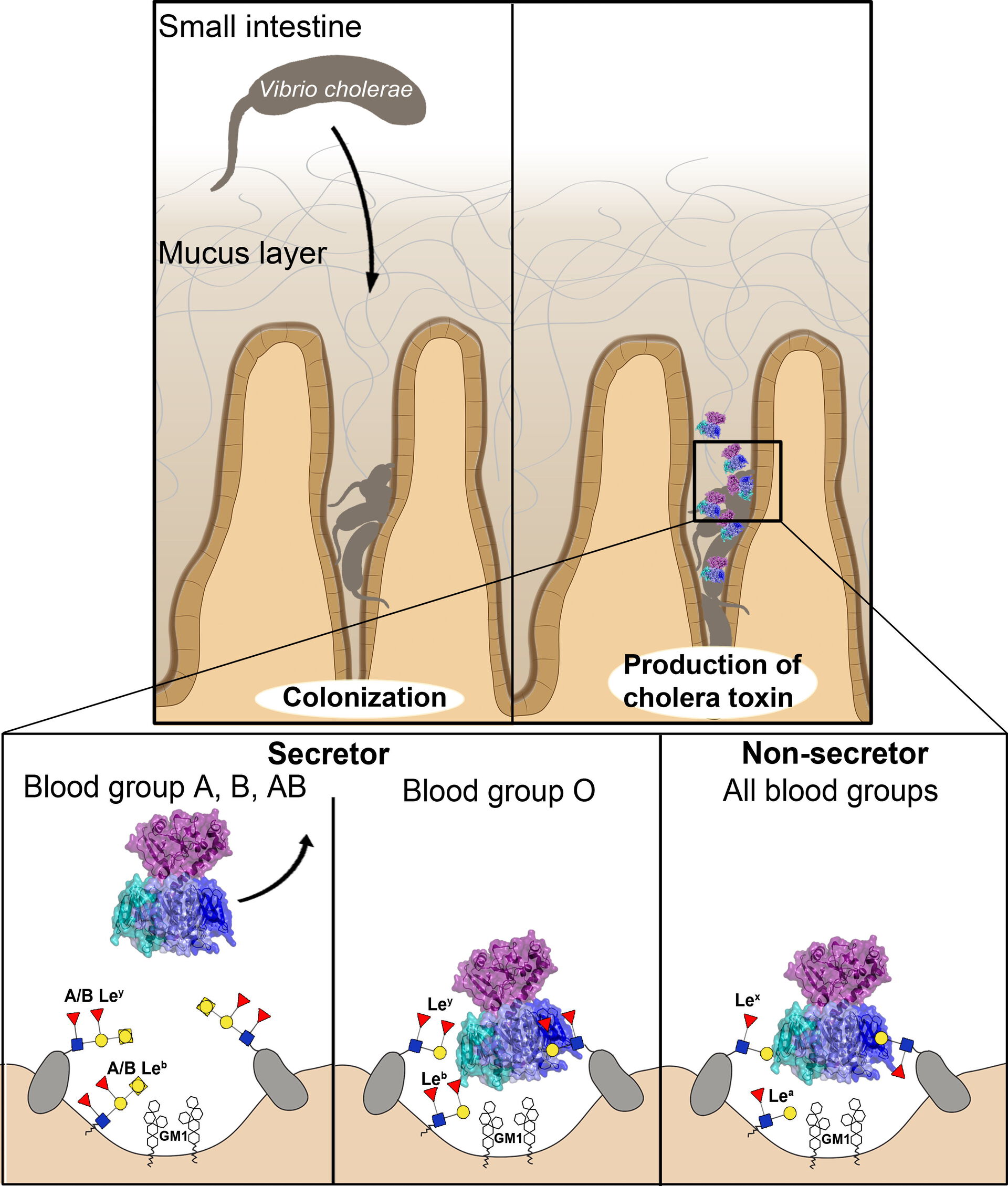
Recently, we also started to understand why cholera is a much more severe disease compared to traveller's diarrhea caused by ETEC infections, namely because CT can be more easily disassembled by the chaperone PDI (Serrano et al., 2022). This work involves a close and highly valued collaboration with the group of Ken Teter at UCF.
Bacterial colonization factors
Using an integrative structural biology approach, we are investigating how adhesins such as GbpA colonize different surfaces, and which conformational changes may be involved. This work on LMPOs involves the close collaboration with the laboratory of Gustav Vaaje-Kolstad at NMBU. Exciting recent results revealed that these proteins have more than one role, and are also actively involved in interfering with the host's immune system (Askarian et al., 2021).