Chorismate mutase (EC 5.4.99.5) plays an important role for the biosynthesis of aromatic amino acids. Located at the branchpoint of the shikimate pathway, this enzyme channels amino acid precursors to the biosynthesis of phenylalanine and tyrosine and away from that of tryptophan. In many organisms, chorismate mutase is important for regulating the balance of aromatic amino acids in the cell.
About the project
Chorismate mutase catalyzes the rearrangement of chorismate to prephenate. This reaction is formally a Claisen rearrangement, an industrially important reaction from the same family as the popular Diels-Alder reaction. Chorismate mutase is the only well-characterized enzyme that catalyzes a pericyclic process and has thus generated considerable interest in the bioorganic circles. Despite extensive studies, however, the enzyme mechanism and in particular the million-fold rate acceleration by chorismate mutase have remained poorly understood.
Moreover, since the shikimate pathway and hence chorismate mutase only exist in bacteria, archaea, fungi and higher plants, the enzyme has a strong potential for the development of herbicides as well as anti-bacterial and anti-fungal therapeutics.
This project builds on a long-standing collaboration with the group of Peter Kast at ETH, Zurich, an expert in directed evolution (see e.g., Fahrig-Kamarauskaitė et al., 2020). In addition to investigating the catalytic mechanism of traditional members of the chorismate mutase family, we have recently turned our attention to two peculiar chorismate mutase subclasses, AroQγ and AroQδ.
Research Highlights
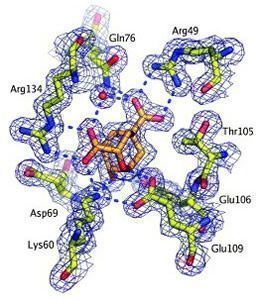
A strategically positioned cation is crucial for efficient catalysis by chorismate mutases (Kast et al., 2000). In collaboration with Don Hilvert's lab, we have replaced this cation with a non-natural amino acid that lacks a charge (arginine to citrulline) in Bacillus subtilus chorismate mutase, the prototype of the AroH family. This allowed us to slow down the reaction by more than 4 orders of magnitude and trap the substrate complex, revealing a movie of the reaction (see animation below; Burschowsky et al., 2014).
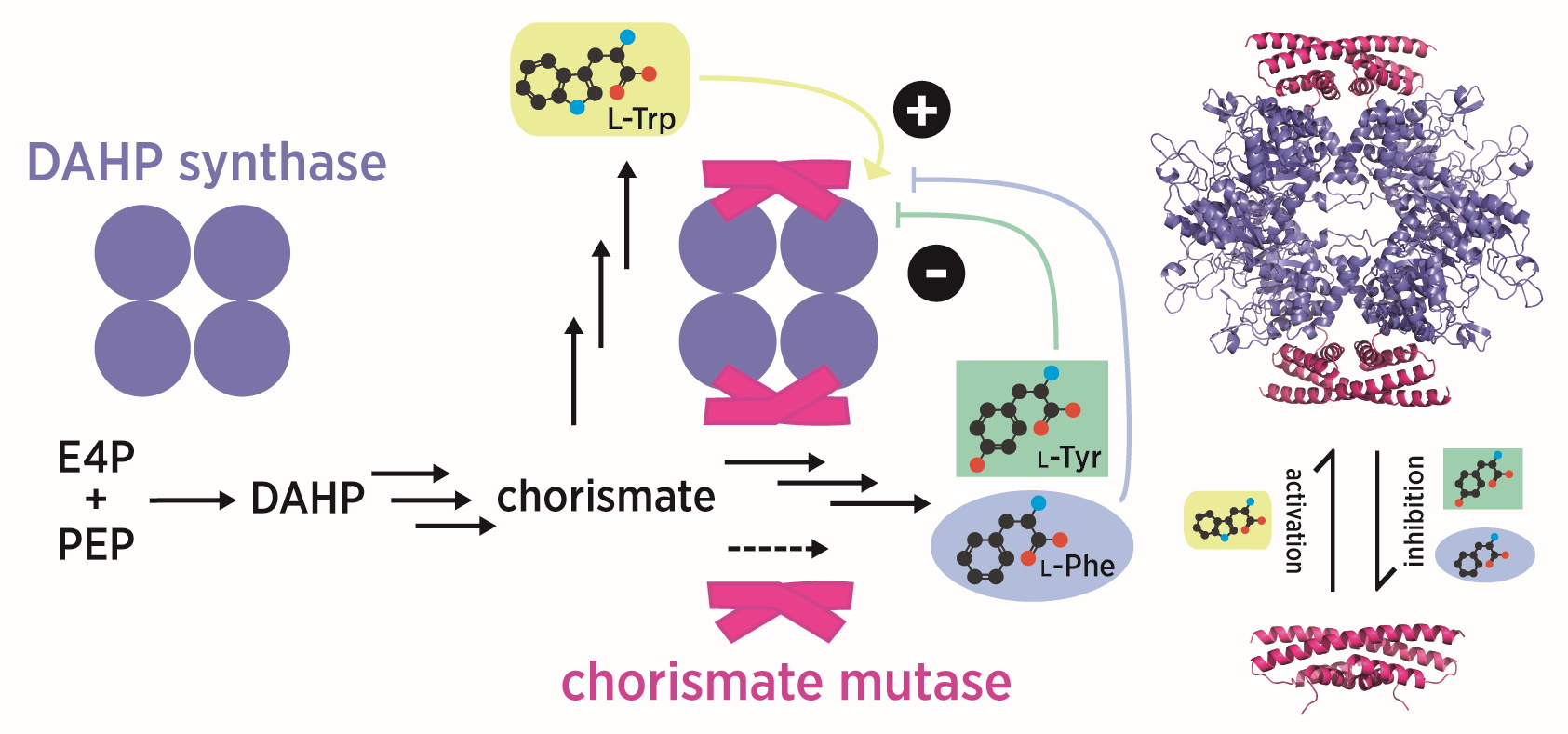
Fascinatingly, not all chorismate mutases are efficient enzymes in nature. Mycobacterium tuberculosis, for example, contains two chorismate mutases: an internal enzyme, which is marginally active, and a secreted enzyme that appears to equip the bacteria with pathogenic properties. The secreted enzyme (MtCM*) exhibits an interesting new fold topology explaining the extreme stability of this enzyme, while the internal, house-keeping enzyme (MtCM) is more similar in structure to the AroQ prototype enzyme from Escherichia coli, EcCM. While MtCM has rather low activity on its own, its catalytic efficiency increases >100-fold on addition of DAHP synthase (MtDS; Rv2178c), another shikimate-pathway enzyme (Sasso et al., 2009). The structure of the MtCM-MtDS complex suggests how key active site residues of MtCM are repositioned upon interaction with MtDS, to rescue its activity (Sasso et al., 2009).
As it turns out, the chorismate mutase activity of the complex, but not of MtCM alone, is inhibited synergistically by phenylalanine and tyrosine (Sasso et al., 2009). Complex formation thus endows the shikimate pathway of M. tuberculosis with an important regulatory feature, with DAHP synthase serving as a regulation platform, killing two bird with one stone (Munack et al., 2016).
Publications
- Burschowsky et al. (2014) Proc Natl Acad Sci U S A. 111, 17516-21
- Fahrig-Kamarauskaitė et al. (2020) J Biol Chem. 295, 17514-34
- Kast et al. (2000) J Biol Chem. 274, 36832-8
- Munack et al. (2016) J Mol Biol. 428, 1237-55
- Ökvist et al. (2006) J Mol Biol. 357, 1483-99
- Sasso et al. (2009) EMBO J. 28, 2128-42