Lectins serve as model systems for protein-carbohydrate interactions. This is true in particular for the large family of legume lectins. In chimerolectins, a lectin domain is coupled to an enzymatic domain. This allows the protein to home in on a target and modify it.
About the project
We were originally interested in legume lectins, with a special focus on the fine specificity difference between the two highly homologous lectins from Erythrina cristagalli (ECL) and Erythrina corallodendron (ECorL) (Svensson et al., 2002). More recently, we turned our interest towards mushroom lectins, among them Marasmius oreades agglutinin (MOA), Polyporus squamosus lectin 1a (PSL1a) and Lyophyllum decastes lectin (LDL) (van Eerde et al., 2015). Many mushroom species are toxic and a significant number of mushrooms possess tonic and medical attributes, which are often related to lectins.
Research Highlights
MOA is one of only few lectins that recognize blood group B antigens, and therefore of interest for blood typing. Moreover, the carbohydrate specificity of MOA resembles that of some bacterial toxins, which induce Hemolytic-Uremic Syndrome (HUS)/Thrombotic Thrombocytopenic Purpura (TTP), a severe human disease that can lead to thrombosis, kidney failure and cortical necrosis; and experiments have demonstrated that MOA can induce damage of the kidney glomerular epithelial cells in mice, which resembles the pathological patterns of HUS/TTP in humans (Warner et al., 2004).
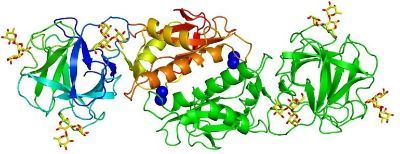
We determined the crystal structure of MOA in complex with the blood group B trisaccharide (Grahn et al., 2009; see figure above) as well as with a xenotransplantation epitope (Grahn et al., 2007). Unexpectedly, we found calcium bound to the protein dimerization interface, close to a structural motif suggesting enzymatic activity (Grahn et al., 2007 and 2009). Subsequently, we could prove that MOA has proteolytic activity (Cordara et al., 2011). We elucidated the catalytic mechanism of MOA and its ortholog PSL1A in detail, including tyrosine gating and trapping of a peptide fragment in flagranti (Cordara et al., 2016 and 2017; Manna et al., 2020; see Figure below).

Now, we are turning our focus to other interesting fungal lectins.
Cooperation
The lectin projects involved the close collaboration with the research groups of Nathan Sharon, Weizmann Institute, Rehovot, Israel (Erythrina lectins) and Irwin Goldstein, University of Michigan, Ann Arbor, Michigan, U.S.A. (mushroom lectins). Our most important active collaboration is with Markus Künzler, ETH, Zurich, Switzerland.
References
Krengel & Imberty (2007) Crystallography and lectin structure database, In: Lectins: Analytical technologies (C. Nilsson, Ed.), Elsevier, pp.15-50.
Manna et al. (2020) Curr Res Struct Biol. 2, 56-67